
|
|
|

|
|
|
 |
Fill On line
Step 1: Fill entry form
Step 2: Preview
Step 3: Give your email
Step 4: Save or Submit
Fill Off line
Step 1: Download form
Step 2: Fill form
Step 3: Save as html
Step 4: Submit
|
 |
 |
 |
 |
|
|
|
|
Electroformation of Giant Unilamellar Vesicles |
Implementation |
|
Interpretation
|
|
|
|
|

|
|
GUV, Giant Unilamellar Vesicles, electroformation
|

|
|
This technique allows to form giant vesicles (from 1 to 100 um) in a couple hours with a good yield and few multilamellar ones. It relies on the hydration of dry films of lipids under an oscillating electric field.
Typically a standard wave genrator is used to apply +-1V at 10 Hz between to electrodes onto which a thin film of lipids has been dried.
It is easy to implement and requires specific adjustments only for certain types of lipids
(higly charged or high melting temperature ones)
|

|
|
Usual measurement |
Exotic measurements
|
|
- Create GUV filled with a non conductive medium
Comments
|
- Allows incorporation of active membrane proteins
- May be adapted to create vesicles filled with salt solution by exchange of buffer during the vesicle growth
Comments
|

|
|
Vesicle size: 1-100um
Applied voltage: 1 V 10Hz
Typical formation time: From 2h to 12h
Max salt: 10mM NaCl Comments
|

|
|
|
|
Materials:
- Wave generator
- ITO coated coverslip
- Glass pipette
- Lipids
- Copper tape
- Desicator under vaccuum
- Chloroform
- Possibly acetonitril or methanol
Comments
|
Providers:
- HP, Agilent, tecktronics...
- ACM
- Hamilton, VWR
- Avanti polar lipids, Sigma...
Comments
|
|

|
|
This method is called electroformation or the bulgarian methods due the the first autors that described it.
Giant vesicles can be easily prepared from mix of PhosphatidylCholines as EggPC.
Other PC, PS, PG, or PE can be used either pur or as a defined mix.
You first need to disolve them in a solvent that will present the 3 following propoerties:
- disolve equally well all lipids,
- evaporate quickly
- wet the ITO coated glass.
Typically people use Pur Chloroform, 95%Chloroform+5% methanol, 95% chloroform+5% acetonitril
- Dilute the lipids at about 1 mg/ml in the solvant
- Prepare the Two ITo coated slides by gluing a piece of copper tape on the conductive side in order to allow the application of voltage difference.
- Rince the ITO surfaces with ethanol then Chloroform to clean it. Vacuum dry for 10 minutes.
- Over an area of about 1 to 2 cm diameter deposit 10 ul of the lipids in solvent using a glass pipette and metal needle. The layer must be as thin and as homogeneous as possible. Hence the need for a slow deposition and a wetting solvant.
- Vacuum dry the slides for at least 1h 30 minutes.
- Using PDMS, Sephadex, Patafix or any kind of soft plastic or wax make a little chamber around lipid area. Typically the gap of the chamber is 1 to 2 mm. Hold the two slides together with binder clips
- Fill the chamber with the inner buffer for the vesicles. Typically 100mM Sucrose. This buffer must contain as few ions as possible
- Apply a sine wave of about 2 Vpp at 10 Hz for 2 to 12 hours. Theses values can be optimized according to the chamber thickness and the solution viscosity.
- Vesicles growth can be monitored through the ITO glass using a phase contrast or DIC microscope.
- To detach the vesicles from the surface apply about 3Vpp square wave 5Hz for an hour.
- Slowly pipette the vesicles out of the chamber using a glass pipette to avoid bursting.
- Transfert them into a 100mM glucose solution to enhance the contrast between the inner and outer medium
Comments
|

|
|
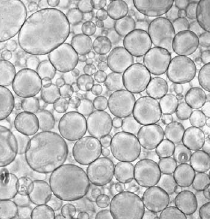
Figure 1: Phase contrast picture of EggPC vesicles grown by electroformation.
|

Figure 2: ITO coated slide. A cylinder of wax (sephadex or patafix) can be put around the lipids to form a chamber. PDMS or soft foam can also be used
|
|
|
|
 |
|
- One can also incorporate proteins in the vesicles by gently prehydration of the film (Girard et al 2004)
- Better yield can be obtained by spin coating the lipid film (Este et al CSB 2005)
- Up to 2M KCL can be introduced in the vesicles by exchangind an equiosmolar glycerol solution with the salin one during the groth process ( Este et al BBA 2005) Comments
|

|
|
- Vesicle Formation by swelling
- Vesicle Formation from emulsion
Comments
|

|
|
- M. Angelova, S. Soleau, P. Meleard, J.F. Faucon, P. Bothorel,
Preparation of giant vesicles by external a.c. electric fields.
Kinetics and applications, Prog. Colloid Polym. Sci. 89 (1992)
127– 131.
- M.I. Angelova, D.S. Dimitrov, Faraday Discuss. (1986) 303.
-P. Girard, J. Pecreaux, G. Lenoir, P. Falson, J.L. Rigaud, P. Bassereau,
A new method for the reconstitution of membrane proteins into giant
unilamellar vesicles, Biophys. J. 87 (2004) 419– 429.
- Estes, D. J. and M. Mayer (2005).Electroformation of
giant liposomes from spin-coated films of lipids.Colloids
and Surfaces B-Biointerfaces 42(2): 115-123.
- Estes, D. J.
and M. Mayer (2005), Giant liposomes in physiological buffer
using electroformation in a flow chamber; Biochimica Et
Biophysica Acta-Biomembranes 1712(2): 152-160. Comments
|
|





 espci.fr
espci.fr